Ratios are the true indicator of the correctness of the fertilisation programme. When designing a fertilisation programme, it is critical to know all the sources of nutritional elements available to a plant, and what those ratios and concentrations are. By knowing these, the rest is a question of mathematics.
Ratios
If the grower is using a medium that has a starting fertility ratio of NPK, of 0 – 1 – 0.5, and the plant shows a total tissue ratio of 4 – 2 – 3, then the grower will have to add a ratio of 4 – 1 – 2.5 to get the correct level of nutrients needed. However, it must be remembered that this assumes perfect values of pH, temperature, and across the growth cycle, and the values are seldom perfect.
Plants seldom take up nutrients equally and they will influence the root zone to acquire more of the nutrients that they need at any given time. These needs will change slightly as they develop, while tissue analysis only gives a snapshot picture of what is happening in the plant. Tissue taken at the end of the crop cycle will show the cumulative value of all the various stages and will not reflect how a plant absorbs these nutrients over time. Juvenile plants take up a different ratio of nutrients to flowering plants; when a plant is preparing to seed, it will begin to accumulate phosphates.
In a situation where all the nutritive elements are correct except one, which is low, then the low element will be the limiter. When this is a minor element (or micro element) that is only needed in a few processes, say sodium for example, then the effect, although real, will be minimal. In a situation where a major element is the limiter, say phosphorous, then the effect can be dramatic because all those compounds made from phosphorous will be incomplete, and those processes that depend on phosphorus will not occur, including vital functions like nutrient uptake, transport, and conversions. By applying sufficient concentrations of these elements in the correct ratio, and providing the right environment, there will be no limiting agent and growth will proceed at the maximum rate that is genetic possible.
It is important to apply sufficient concentrations of these elements, but it is also important not to apply too much; and here, both high concentrations and incorrect ratios can play a role. Just because there is a shortage of one nutrient does not mean the plant will stop itself absorbing the other elements provided. These unused elements usually find their way into the vacuole of the cell and remain there. Vacuoles not only provide water storage and structural support, they also serve as garbage dumps. Heavy metals will accumulate there, such as copper, boron, molybdenum, and manganese, and these can cause issues in any animals that then consume the plants. Plants will also accumulate non-nutritive elements such as lead and uranium if they are present in an available form in the root zone. Where not enough ATP is available to totally convert nitrates into usable N, then nitrites can accumulate. Excess ammonium shunted into the vacuole converts to nitrites and nitrosamines, a cancer causing agent. Keeping these ratios close, while avoiding limiting values, is the ultimate goal of a plant nutrition programme, and the best way to keep consumers consuming.
Plant needs
So what does a plant need in the way of phosphorus, how do we provide this, and what can we expect over time and development? The best way to know a plant’s needs is to know what makes up the plant and the ratio of these elements within the plant. Once you know this, and you have some information about the substrate, it is fairly easy to apply this ratio by using several known fertilising materials. However, it is also important to monitor these values at the various stages of growth and to make adjustments at each stage as required.

The other way is to use a product that was designed on the basis of the plant itself after thorough research done manufacturer (a ‘complete fertiliser’), and adapted to the substrate involved. Care must to be taken by the grower to ensure that all the variables correct, such as pH and temperature, or at least to follow the manufacturer’s guidelines. It is equally important to use the substrate that the fertiliser was designed for, since the substrate can cause the ratios discussed earlier to change once applied. The grower must be sure to follow the manufacturer’s guidelines closely, taking care not to substitute products because most will provide different levels of the components or a different form.
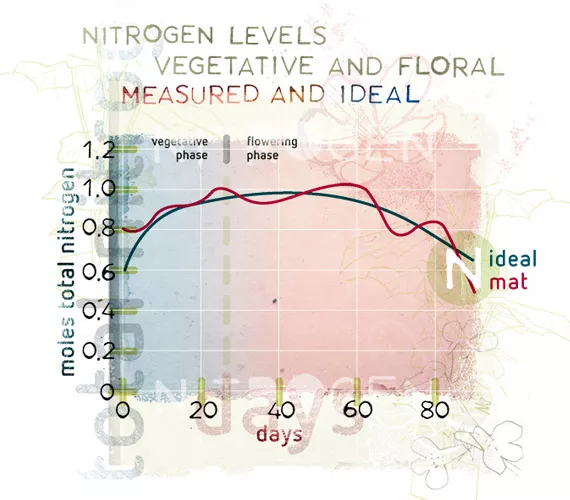
What is equally important, from the nutrient balance point of view, is to provide the ratio that the plant needs, when it needs it. A plant’s need for phosphorus goes up during the earlier stages of flowering then falls back; but still its appetite for phosphorus has been escalating throughout the plant’s development. This is known as the phosphorus utilisation curve, which on a graph looks like a bell curve. The only additional phosphorus that a grower needs to apply is any extra amount that the plant requires that is not provided by the main fertiliser, and that will vary over time: the ratio game again. The plant will change the environment around the root zone in order to activate phosphates to make them available to the plant. The total need for phosphorus in the root zone will ultimately be based not only on the needs of the plant, but also with the level of activity that the environment will have on the ultimate availability of phosphate.
Special Considerations
The Phosphorus Pathway in the plant is wide and all encompassing. Phosphorus starts out in the seeds at high levels to ensure the plant has enough to initiate all the metabolic processes it will require as well as the growth processes. ATP is used to build structure, chemical compounds, and take up the other elements needed for these processes. More phosphorus is found in the root tissues because relatively large amounts are needed to move nutrients into the plant and into the transport pathways. It is turned into ATP for local use or transported to other cells to be transformed into ATP or used directly as ATP (assimilated). Once it is the form of ATP or one of the other energy components, it is released for energy by way of many activities and is then free to be used in the formation of other phosphorylated compounds. It can also be converted back into ATP. More phosphates are also found in the flowers themselves because of the decrease in locally produced ATP and because the plant accumulates phosphorus for the seeds and other energy-intensive requirements of the flower tissues such as pollen production.
In some substrates, such as mineral soils, roughly 50% of the phosphates applied are rendered immobile and fixed permanently in the medium. As a result, more needs to be added to the medium, so while the amount added is higher, the amount realised is lower. Plant mediums that have active micro-life will also see a depletion of the available phosphorus occur, because it is consumed by the microlife who also use ATP. The pH of the soil solution will affect the available phosphorus, as will temperature and overall concentrations of other elements such as potassium, a synergistic effect which is also a ratio issue. The grower needs to be aware of all these variables when designing a nutrient programme for the crop. Most nutrient lines are designed with this line in mind: in other words, the ratio, composition, source, and application rate of each component product adds to the final ratio of every nutrient that would be required by the plant.
The marketing effect
The noise level about phosphorus amounts to just that - noise. Numbers on fertilisers are legitimate in most incidents, especially where regulations are in place. The numbers are not wrong and all they do is to indicate the concentration of the constituent elements. The type or source of these elements can be the essential factor in determining the final availability of the nutrients based on the overall system. The grower is the final determining factor in how much to use. Complete fertilisers are designed to provide the correct ratio of the elements required when the entire line is mixed according to instructions; the concentration is affected by the application rate.
The problem with phosphorus is knowledge, and old legislation relating to how the element must be measured and reported. When viewed correctly, phosphorus should be in the correct range as adjusted for the root zone environment. Knowing how to read and accept both the labels and reported findings, and interpret the data, is crucial in determining the truth behind the advertising and statements made about products and results.
In the end, all this noise is about marketing. Some companies will produce products without putting much thought into their composition, selling them as more than what they are - simple fertilisers. Some companies will take advantage of a confusing situation and lack of knowledge to promote a myth and tout a weaker product. Other companies actually do their research and know what standards they are designing too, understand the relationships that influence nutrient availability, and actually design a product line that works together.

What to look for
What should growers be looking for? Firstly, a grower must decide if they are going to use an off-the-shelf version or a complete fertiliser, or mix their own. Off-the-shelf products must be designed for a particular plant and the particular growing method that will be used. Mixing your own fertiliser requires extensive knowledge of chemistry and horticulture and is generally not the best method for growers of smaller commercial operations or hobbyists.
Phosphorus can be applied in many formulations on the basis of the base mineral that it is derived from. Base minerals are also associated with other elements, some good, and some not-so-good. For example, monopotassium phosphate with an NPK ratio of 0 – 10 – 11 (commercial) and 0 – 4.4 – 9.13 (elemental) includes beneficial potassium too. Sodium nitrate, meanwhile, with an NPK ratio of 15 – 0 – 0, provides beneficial nitrates, as well as sodium (which is less beneficial).
The bigger the phosphorous number, the less of it will actually get used. Make sure that the number is small enough to not make costly mistakes when applying smaller measured amounts. Bigger numbers may or may not bring down the unit cost of phosphorus since it is based on a different mineral which sometimes has other more costly materials attached. Diammonium phosphate has an NPK of 21 – 53 – 0, but the nitrate is expensive and the composition of the product means that it needs some balancing with other components and care in application because it forms an acid very easily. Using an off-the-shelf version will probably offset most of these issues and result in an easier application process. The grower should be aware of two issues: the first is nutrient contamination and the second is the fact that nutrient sources will always vary in characteristics and availability. Nutrient constituents can become contaminated with other elements during either the mining or the manufacturing process. Contaminants such as lead or other heavy metals can accumulate in the plant and cause damage to the plant or to consumers. Some nutrient constituents can have adverse effects on pH, be less soluble and therefore less available, or can come in a less than optimal form. The ammonium ion, although it is an acceptable source of nitrogen, becomes less acceptable as the concentration increases to the point of toxicity. So growers should look for nutrients that are high quality, clean, and well formulated. Find or request a heavy metal analysis for the nutrient line before use - this will show how clean a fertiliser is.
For complete fertilisers, the grower should deal with a quality company that has their success in mind, that does research when developing its products in a legitimate manner, and that maintains high quality standards. This holds true for both approaches to fertilising crops. For complete fertilisers or fertiliser lines, the company should also understand all the relationships that affect the provision of nutrients to the plant and should never ever attempt to sell their products based on shortcomings in the knowledge of the consumer.
A good company will educate its consumers and stay true to the science. Companies with an overactive marketing department and no research department will, out of necessity, seek sell the glitz by straying into the realms of science fiction.
Bibliography
- Brady, Nyle C., and Ray R. Wells. The Nature and Properties of Soils. 13th . Upper Saddle River, NJ: Prentice Hall, 2001.
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives. New York: Wiley, 1972.
- Epstein, E. “Silicon.” Annu. Rev. Plant Physiol. Plant Mol. Biol. 50 (1999): 641-664.
- Paul, E. A., and F. E. Clark. Soil Microbiology and Biochemistry. 2nd. San Diego: Academic Press, 1996.
- Plant Research, B.V., interview by Geary Coogler. Conversations on Phosphorous Utilization Oosterhout, (October 27, 2009).
- Schwarz, A., W. Wilcke, and W. Zech. “Heavy Metal Release from Soils in Batch pH(stat) Experiments.” Soil Sci. Soc. Am. J. 63 (1999): 290-296.
- Taiz, L., and E. Zeiger. Plant Physiology. 3rd. Sunderland: Sinauer Associates, Inc., 2002.
- Yamagata, M., and A. E. Noriharu. “Direct Acquisition of Organic Nitrogen by Crops.” JARQ 33, no. 1 (January 1999): 15-21.








